
Clinical trial inspections, monitoring visits, and audits are all important quality assurance measures that are used to ensure the integrity of clinical trials. However, there are some key differences between these three activities that are important for researchers to understand. By taking some time to learn more about them and how to prepare for them, you can help to ensure that your trials are conducted smoothly and efficiently, and that the rights and safety of human participants are protected.
Here’s an overview of the differences:
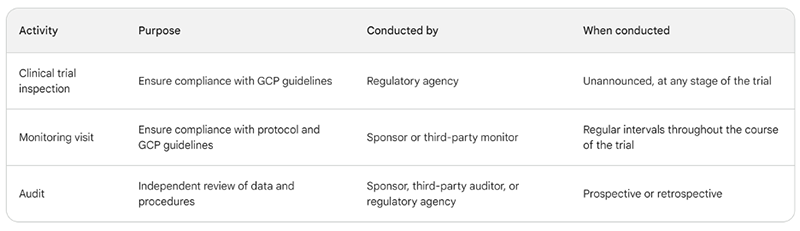
- Clinical trial inspections are conducted by regulatory agencies, such as the FDA, to ensure that clinical trials are conducted in compliance with Good Clinical Practice (GCP) guidelines. Inspections can be either for-cause or not-for-cause. They are typically unannounced and can occur at any stage of a clinical trial. Note – an inspection is often referred to as an onsite audit, often by regulatory authorities.
- Monitoring visits are conducted by the sponsor of a clinical trial, or by a third-party monitor, to ensure that the trial is being conducted in accordance with the protocol and GCP guidelines. Monitoring visits are typically scheduled at regular intervals throughout the course of a trial.
- Audits are independent reviews of the data and procedures used in a clinical trial designed to provide assurance that the trial is being conducted in an ethical and compliant manner. Audits can be conducted by a sponsor, third-party auditor, or regulatory agency. Audits can be either prospective (conducted before the trial begins) or retrospective (conducted after the trial has ended).
In general, clinical trial inspections are the most rigorous form of quality assurance. However, monitoring visits and audits can also play an important role in ensuring the integrity of clinical trials. The specific type of quality assurance activity that is most appropriate for a particular clinical trial will depend on a number of factors, such as the stage of the trial, the risk level, and the regulatory requirements.
It’s also important to understand the purpose, frequency, scope, independence:
- Purpose: Monitoring is designed to ensure that the trial is being conducted in accordance with the protocol and GCP guidelines, while auditing is designed to provide assurance that the trial is being conducted in an ethical and compliant manner.
- Frequency: Monitoring is a continuous process that is conducted throughout the course of the trial, while auditing is typically conducted at specific points in time, such as at the start of the trial, at interim analyses, or at the end of the trial.
- Scope: Monitoring typically focuses on specific areas of the trial, such as data collection and safety reporting, while auditing has a broader scope and can look at all aspects of the trial, including the protocol, the procedures, and the documentation.
- Independence: Monitors are typically employees of the sponsor or CRO, while auditors are typically independent third-party organizations. This means that auditors have no vested interest in the outcome of the trial and can provide an objective assessment of the site’s compliance with GCP guidelines.
Next, there are the parties conducting the site review.
- Sponsor: These are conducted by the sponsor of the clinical trial, either the biopharmaceutical, device or digital therapeutic company or the investigator (for investigator-initiated studies). The Sponsor may often contract these activities to a CRO or other independent party. They are typically conducted to assess the overall progress of the trial and to ensure that the site is complying with the protocol and GCP guidelines.
- Regulatory: These are conducted by regulatory agencies, such as the FDA or the European Medicines Agency (EMA). They are typically conducted to ensure that the trial is being conducted in accordance with applicable regulations.
- Internal: These are conducted internally by Quality Assurance (QA) staff, typically independent of site operations. This is more common at larger academic centers, health systems and site networks that have established separate, internal controls for the highest risk studies.
- Independent: These are conducted by independent third-party organizations. They are typically conducted to provide an objective assessment of the site’s compliance with GCP guidelines.
The specific areas that are reviewed will vary depending on the type (audit, monitoring visit, inspection) and the specific concerns of the reviewer. However, some common areas that are audited include:
- Informed consent process
- Data collection and management
- Safety reporting
- Compliance with the protocol and GCP guidelines
- Training of staff
- Maintenance of accurate records
- Clinical trial audits, monitoring visits and inspections are an important part of ensuring the quality and integrity of clinical trials. By understanding the differences and being ready, clinical trial sites can help to ensure that their trials are conducted smoothly and efficiently, and that the rights and safety of human subjects are protected.
Here is a table summarizing the key differences between clinical trial inspections, monitoring visits, and audits:
For more information on how to be ready for an audit, inspection, or monitoring visit, read this article.


