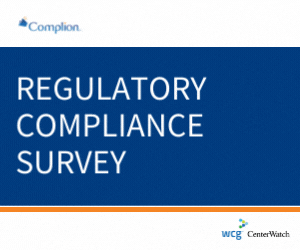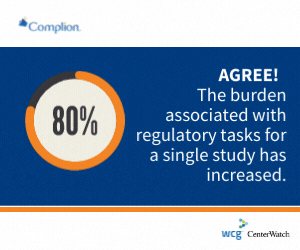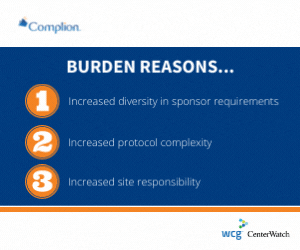
Approximately 80 percent of clinical research sites polled in a recent Complion, Inc.-sponsored survey said the costs and burden associated with administrative tasks for a single study has increased over the past two years, continuing a five-year upward trend.
The survey found that rather than attributing additional compliance rules set by regulators, sites noted the following primary causes:
- Sponsor requirements that vary wildly from study to study;
- Increasing protocol complexity; and
- Multiple amendments.
This aligns with what Complion has heard anecdotally in its conversations with sites. Every sponsor is coming to research sites with different interpretations of regulations, different mandates, and different portals and tools to be used during a study.
Sites often feel they need to comply with what sponsors require. While sponsors may have preferred tools and technologies for handling study data, they often are open to sites using their own systems they have a proven history. Sponsors simply want to ensure the data produced by a study is sound.
There are options that allow sites to take more control. For example, sites can advocate for more standardization among studies.
 More than 40 percent of survey respondents indicated they handle the bulk of regulatory tasks with specialists, with only 34 percent assigning the work to a study coordinator or nurse.
More than 40 percent of survey respondents indicated they handle the bulk of regulatory tasks with specialists, with only 34 percent assigning the work to a study coordinator or nurse.
The most time-consuming tasks noted by survey respondents included:
- Managing study documents (8.8 hrs./wk);
- Initial regulatory file preparation (8 hrs./wk.);
- Managing and reviewing correspondence (5.9 hrs./wk.);
- Document creation (5.4 hrs./wk.); and
- Reviewing documentation (5 hrs./wk.)
Survey respondents noted they spend approx. $15,000 per study on administrative work and are reimbursed by sponsors little more than an average of $10,000. The difference in cost vs. reimbursement is highest for regulatory compliance training of site staff ($1,700 vs. $200); training on a sponsor’s or CRO’s regulatory web portal ($1,400 vs. $400), and maintaining a study archive ($2,400 vs. $1,200).
Survey results indicated that about 19 percent of sites are currently using third-party document management systems to store study documents electronically. Of the sites not currently using an electronic document management system, 80 percent indicated they do not have plans to purchase a system in the near future (2 years). Approximately 63 percent of respondents indicated they send their study documentation to a third party for archival.
Read the complete survey results.
Are you confident in the eReg tools you are using for your studies? Why not begin a conversation with Complion to determine how you can improve your processes. Schedule a demo of our eReg document management platform.


